
FDA ICH指导原则目录.pdf

qw****27



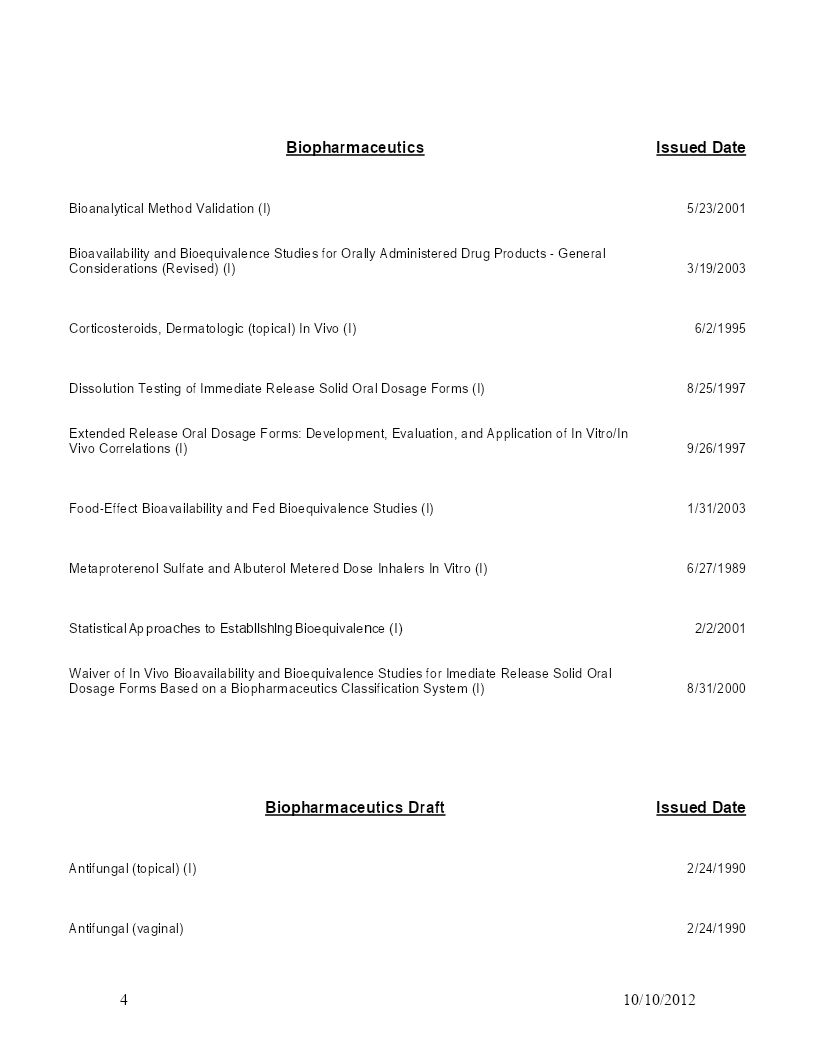






亲,该文档总共52页,到这已经超出免费预览范围,如果喜欢就直接下载吧~
相关资料

FDA ICH指导原则目录.pdf
CenterForDrugEvaluationandResearchListofGuidanceDocumentsGuidancedocumentsrepresenttheAgency'scurrentthinkingonaparticularsubject.TheydonotcreateorconferanyrightsfororonanypersonanddonotoperatetobindFDAorthepublic.Analternativeapproachmaybeusedifsuchappro

ICH指导原则目录.pdf
QualityGuidelines(质量)编号阶段发布日期内容Q1Stability(稳定性)StabilityTestingofNewDrugSubstancesandQ1A(R2)Step52003-2-6Products新原料药和制剂的稳定性试验StabilityTesting:PhotostabilityTestingofNewDrugSubstancesandQ1BStep51996-11-6Products稳定性试验:新原料药和制剂的光稳定性试验StabilityTestingforNewQ1

ich指导原则文件目录.pdf
精选资料人用药品注册技术要求国际协调会(ICH)文件目录ICH的论题主要分为四类,因此ICH根据论题的类别不同而进行相应的编码分类:1.“Q”类论题:Q代表QUALITY,指那些与化工和医药,质量保证方面的相关的论题。Q1/Q2...Q10都属于这类。2.“S”类论题:S代表SAFETY,指那些与实验室和动物实验,临床前研究方面的相关的论题。3.“E”类论题:E代表EFFICACY,指那些与人类临床研究相关的课题。4.“M”类论题:M代表MULTIDISCIPLINARY,指那些不可单独划入以上三个分类的

ich指导原则文件目录.pdf
人用药品注册技术要求国际协调会(ICH)文件目录ICH的论题主要分为四类,因此ICH根据论题的类别不同而进行相应的编码分类:1.“Q”类论题:Q代表QUALITY,指那些与化工和医药,质量保证方面的相关的论题。Q1/Q2...Q10都属于这类。2.“S类论题:”S代表SAFETY,指那些与实验室和动物实验,临床前研究方面的相关的论题。3.“E类”论题:E代表EFFICACY,指那些与人类临床研究相关的课题。4.“M”类论题:M代表MULTIDISCIPLINARY,指那些不可单独划入以上三个分类的交叉涉及

ICH-指导原则文件目录.pdf
-可编辑-感谢下载支持的论题主要分为四类,因此ICH根据论题的类别不同而进行相应的编码分类:类论题:Q代表QUALITY,指那些与化工和医药,质量保证方面的相关的论题。Q1/Q2...Q10都属于这种。2.“S”类论题:S代表SAFETY,指那些与实验室和动物实验,临床前研究方面的相关的论题。3.“E”类论题:E代表EFFICACY,指那些与人类临床研究相关的课题。4.“M”类论题:M代表MULTIDISCIPLINARY,指那些不可单独划入以上三个分类的交叉涉及的论题。1.Q1A(R2):Stabili
