
ACTD required dox vietnam.pdf

xf****65




亲,该文档总共20页,到这已经超出免费预览范围,如果喜欢就直接下载吧~
相关资料

ACTD required dox vietnam.pdf
THEASEANCOMMONTECHNICALDOSSIER(ACTD)FORTHEREGISTRATIONOFPHARMACEUTICALSFORHUMANUSEPARTII:QUALITYTABLEOFCONTENTSScopeoftheGuideline…………………………………………………..2SectionA:TableofContents…………………………………………..2SectionB:QualityOverallSummary…………………………………2SectionC:BodyofD

ACTD目录.docx
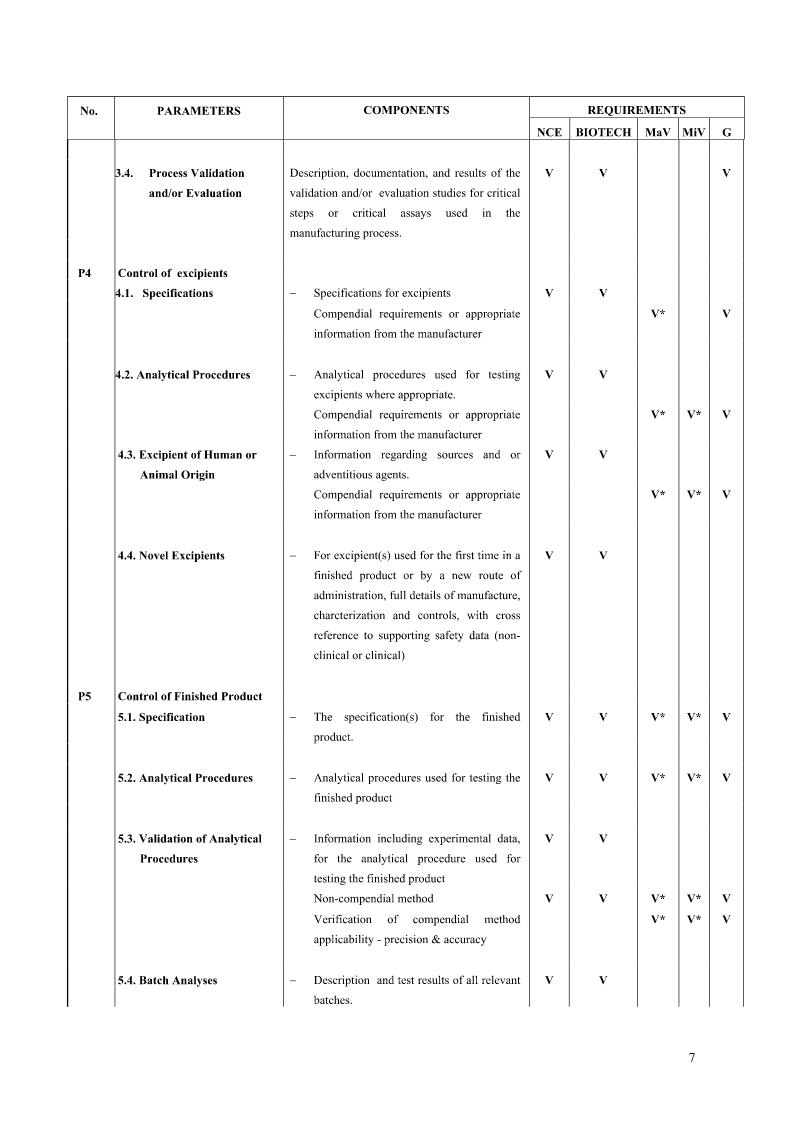
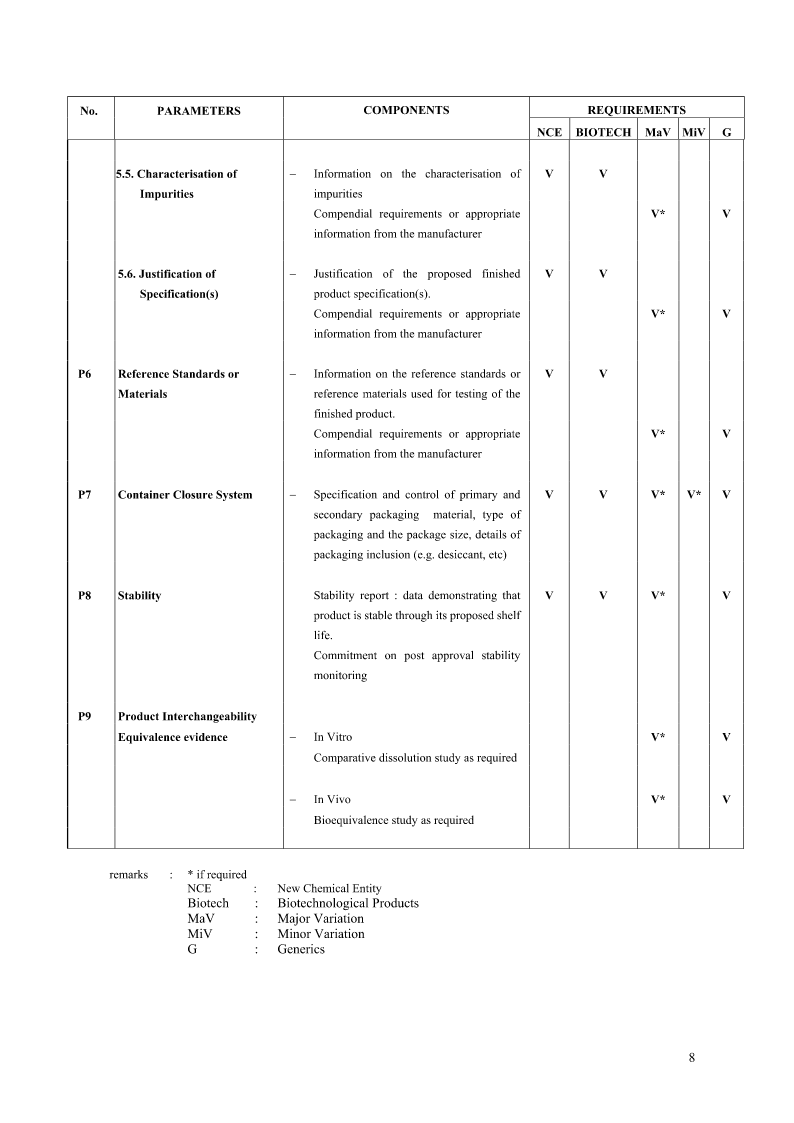
A-DRUGSUBSTANCES1.GeneralInformationS.1.1NomenclatureS.1.2StructuralformulaS.1.3GeneralPropertiesS2.ManufactureS2.1Manufacturer(s)S2.2DescriptionofMfg.ProcessandProcessControlsS2.3ControlofMaterialsS2.4ControlofCriticalStepsandIntermediatesS2.5Processvali

Fosters in Vietnam.doc
Chapter8FostersinVietnamWRETTENBYTUSHARSHARMABackgroundFostersGroupLimited(FGL)isaMelbourne-basedglobalmulti-beveragegroupproducingastrongportfolioofbeer,wine,spirits,ciderandnon-alcoholicbeverages.Fostersisparticularlywellknownfordifferentbrandsofbeersfa

Delegation to Thailand and Vietnam.doc
DelegationtoThailandandVietnamforThe20thJointEconomicCooperationMeetingbetweenCIECAandFTI第二十屆台泰經濟合作會議&The16thJointBusinessCouncilMeetingbetweenCIECAandVCCI第十六屆台越工商聯席會議August4-14,2009TentativeItineraryandProgramAugust4(Tuesday)08:40-11:20Dep

Required and Elective Courses.doc
RequiredandElectiveCoursesThesis:Requiredandelectivecoursesarequitedifferent.OutlineLeadingpartThedifferencesbetweenrequiredcoursesandelectiveonesTheirartificialmeaningsTheirdominantfunctionsandgoalsTheirfinalexaminationsConclusionAsweallknown,there’sapop
