
第二讲溶剂效应.ppt

sy****28



亲,该文档总共58页,到这已经超出免费预览范围,如果喜欢就直接下载吧~
相关资料

第二讲溶剂效应.ppt
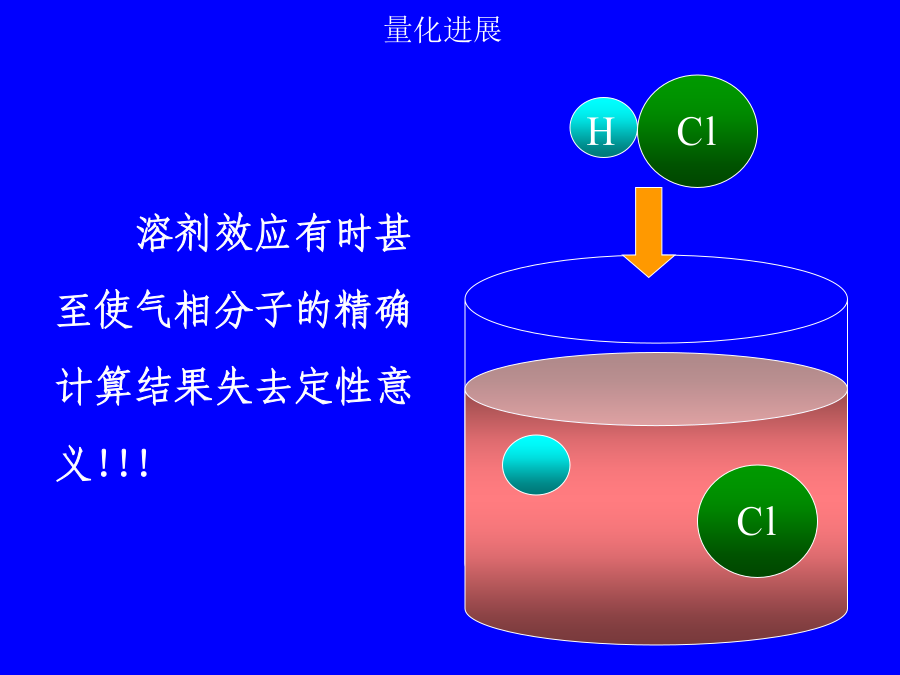
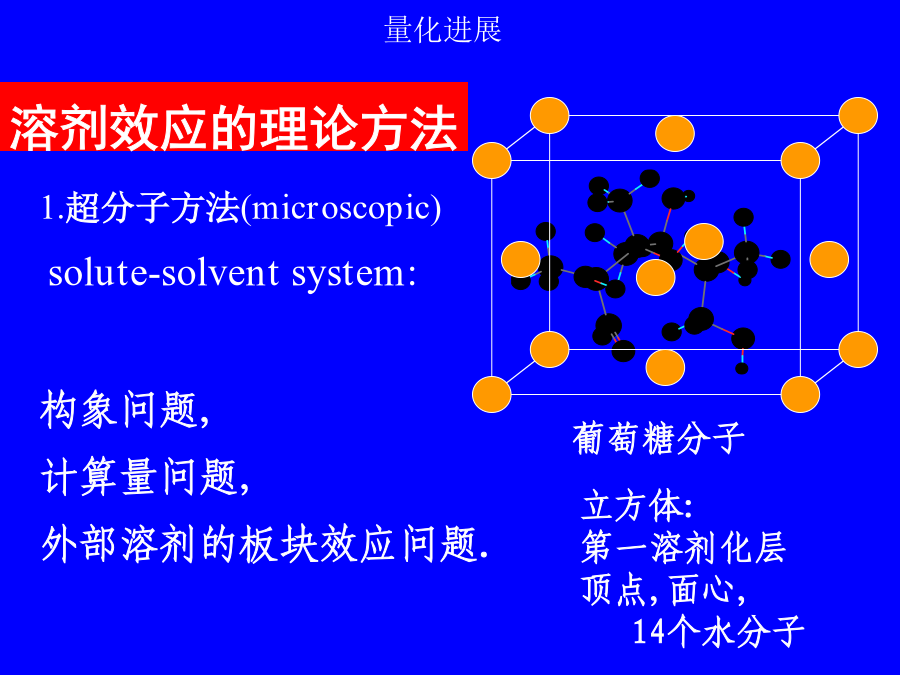
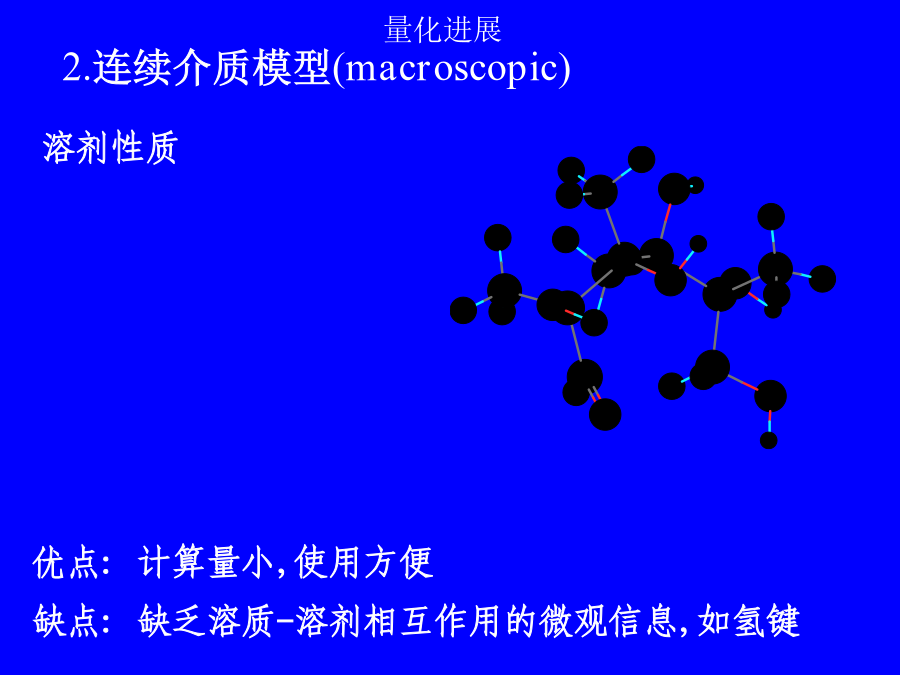
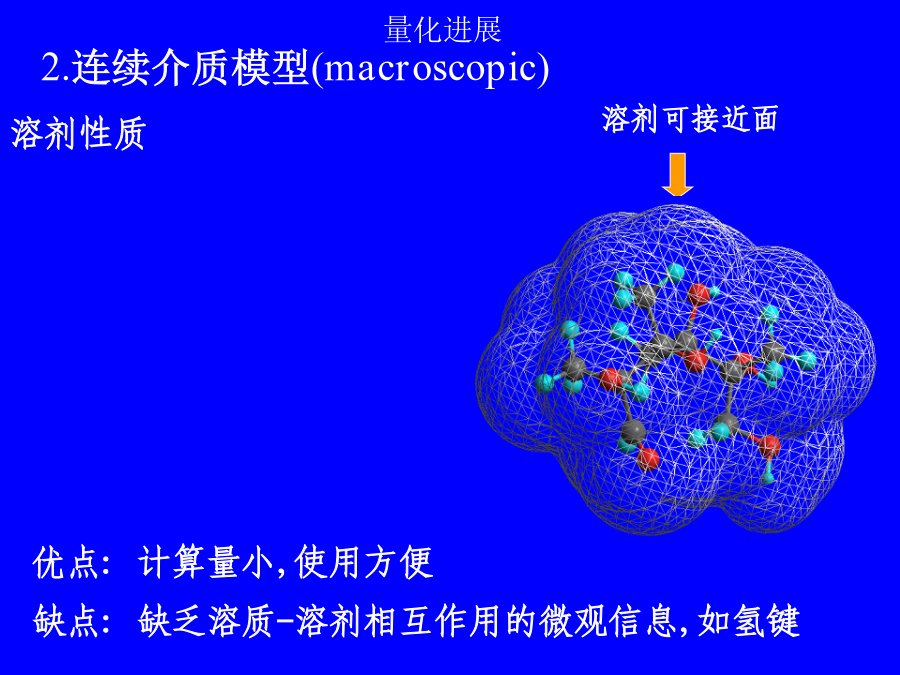
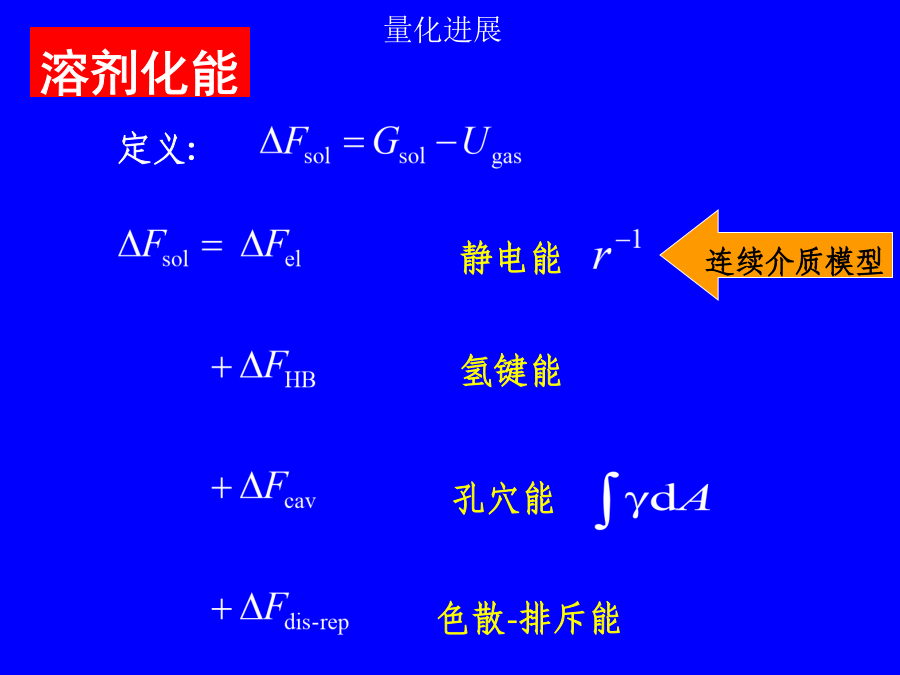
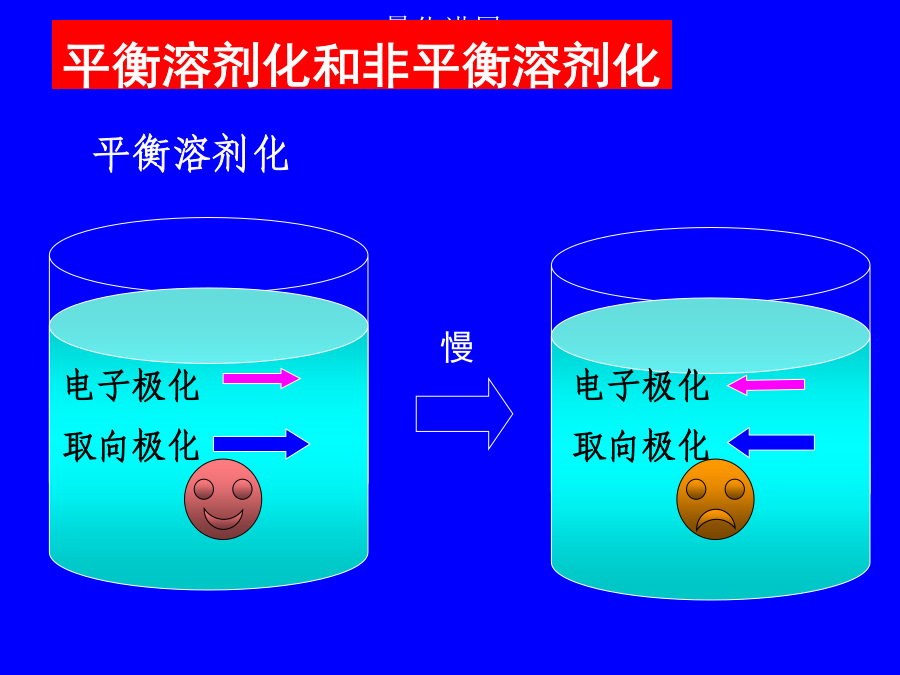
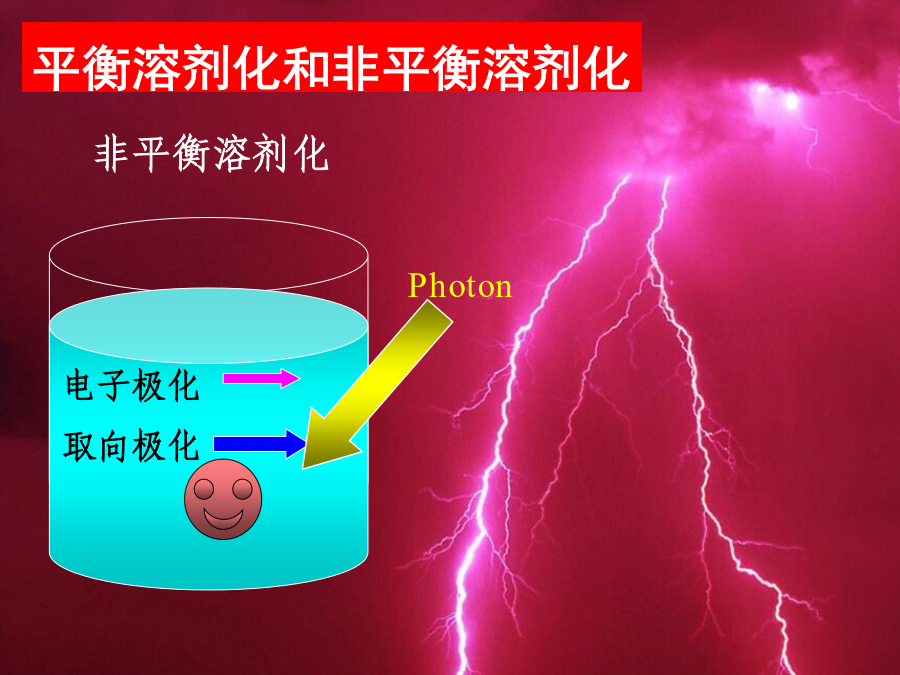
第二讲溶剂效应溶剂效应有时甚至使气相分子的精确计算结果失去定性意义!!!1.超分子方法(microscopic)solute-solventsystem:构象问题,计算量问题,外部溶剂的板块效应问题.溶剂性质介电常数e极化电荷孔穴表面介质无结构各向同性(稀溶液)各向异性(液晶)优点:计算量小,使用方便缺点:缺乏溶质-溶剂相互作用的微观信息,如氢键3.超分子-连续介质方法溶剂化能平衡溶剂化平衡溶剂化和非平衡溶剂化CPL,255,327(1996)1.连续介质模型球孔穴的Born公式(点电荷模型)球孔穴的点偶

吸收带类型与溶剂效应.ppt
*************************

液相色谱溶剂效应.pptx
样品溶剂:纯甲醇什么是溶剂效应?溶剂强度顺序进样体积对色谱行为的影响当样品用流动相溶解时,进样体积对色谱行为影响较小。消除溶剂效应的方法感谢您的观看!内容总结

溶剂效应介绍学习教案.pptx
会计学溶质和溶剂相互作用(zuòyòng)叫做溶剂化。它是指溶液中溶质被附近的溶剂分于包围起来的现象。例如溶质R+L-和溶剂水作用(zuòyòng)的示意图如下:7.1.1根据溶剂(róngjì)的极性分类/溶剂的性质主要(zhǔyào)有:极性、氢键以及酸碱性等。溶质(róngzhì)溶剂极性使速度(sùdù)增加在极性溶剂中略为(lüèwéi)有利其原因是,氢键的形成及由电子对的给予和接受而产生的作用(zuòyòng).比溶利因静电作用(zuòyòng)所产生的分子间作用(zuòyòng)力要大得多。
电子效应和溶剂效应专题培训课件.ppt
取代基效应3.传递有一定限度(短程传递),经过三个碳原子以后,已极微弱。例如-氯代辛酸二.诱导效应的相对强度2.通过分析NMR化学位移确定同周期:电负性随元素的族数增加,故-I效应自左至右增加。Eg.-I:-CR3<-NR2<-OR<-F同一族:元素电负性随周期数增高而递减,故-I效应自上而下降低。Eg.-I:-F>-Cl>-Br>-I;-OR>-SR带正电荷的基团比同类型的不带电荷的基团吸电子能力强得多;Eg.–I:-N+R3>-NR2与碳原子相连的原子,若是同种原子但饱和程度不同,则不饱和程度高的吸
