
原子轨道与分子结构的轨道理论关系.pdf

as****16







亲,该文档总共51页,到这已经超出免费预览范围,如果喜欢就直接下载吧~
相关资料

原子轨道与分子结构的轨道理论关系.pdf
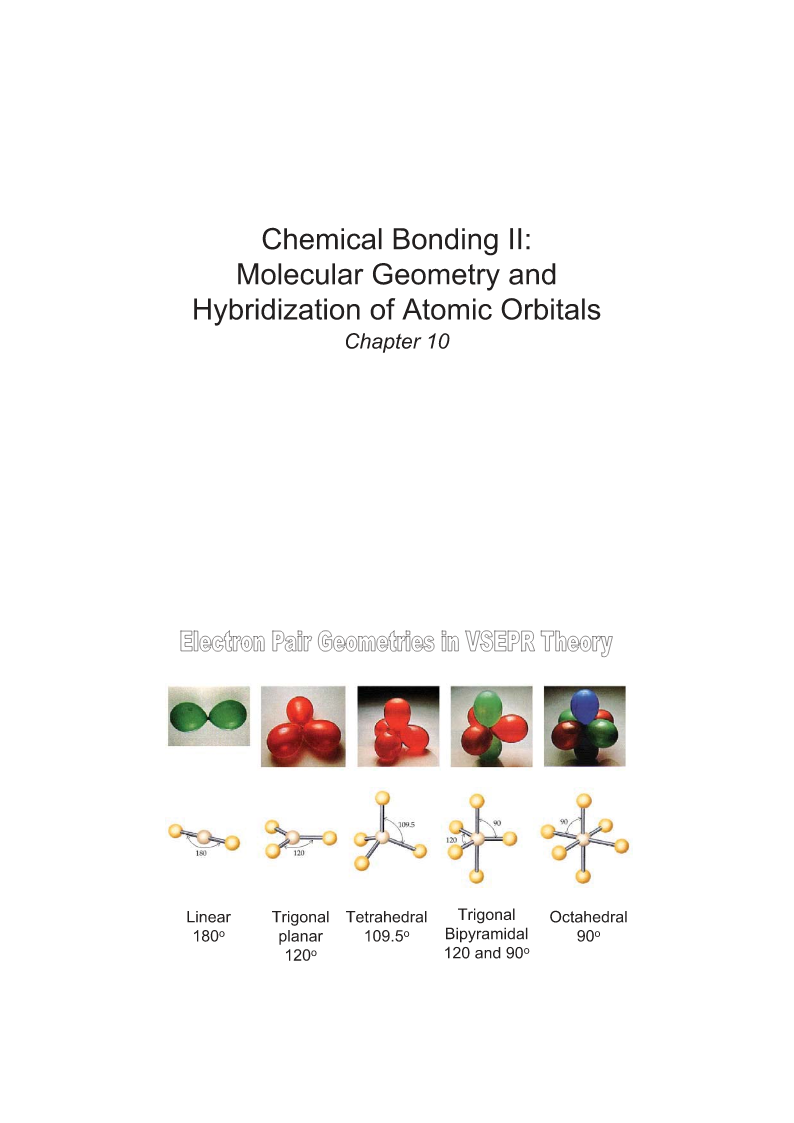
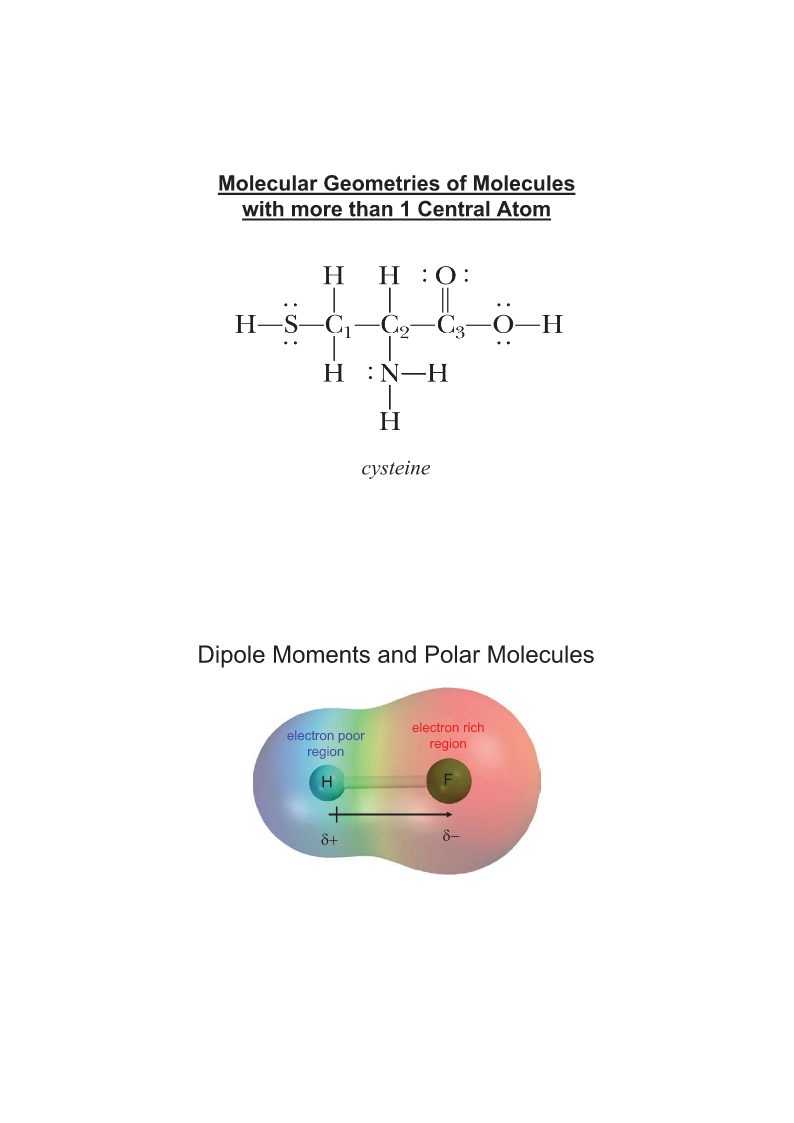
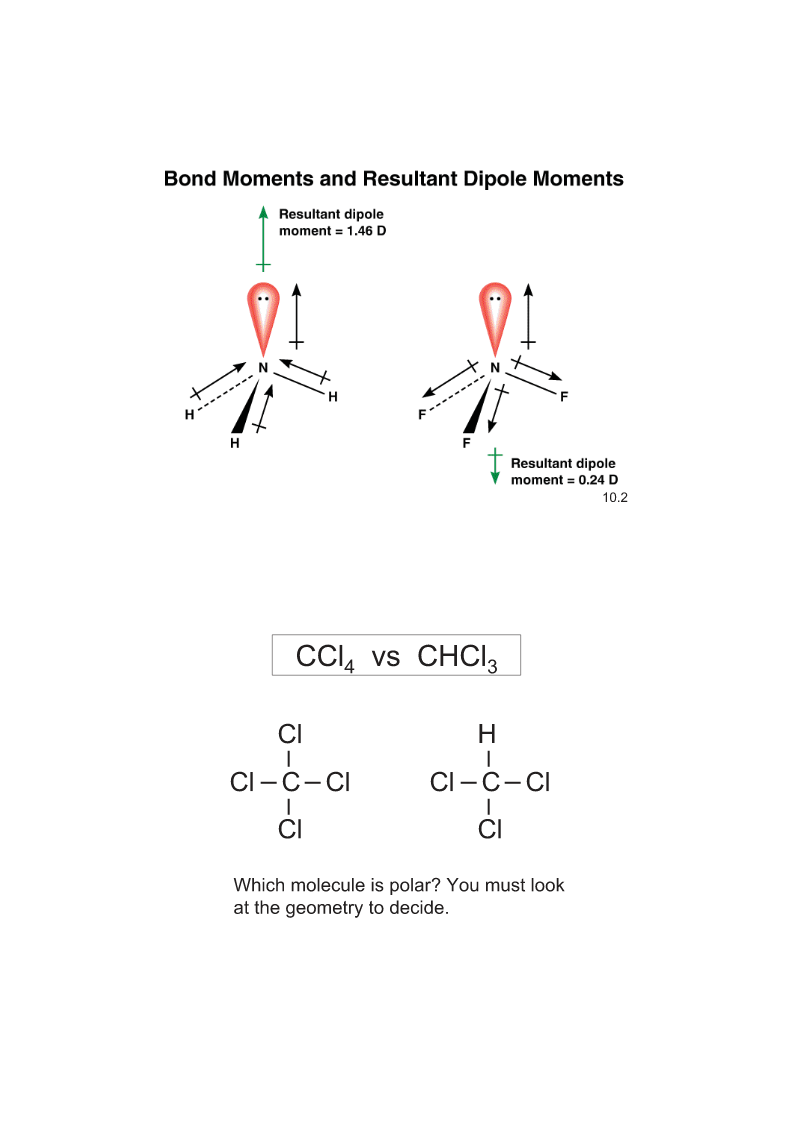
ChemicalBondingII:MolecularGeometryandHybridizationofAtomicOrbitalsChapter10LinearTrigonalTetrahedralTrigonalOctahedral180oplanar109.5oBipyramidal90o120o120and90olinearLinear(AB2)Trigonalplanar(AB3)TrigonalplanarBent(AB2E)TetrahedralPyramidal(AB4)(AB3E)Te

能层与原子轨道.ppt
第一节原子结构与性质[考纲展示]1.了解原子核外电子的排布原理及能级分布,能用电子排布式表示常见元素(1~36号)原子核外电子、价电子的排布。了解原子核外电子的运动状态。2.了解元素电离能的含义,并能用以说明元素的某些性质。3.了解原子核外电子在一定条件下会发生跃迁,了解其简单应用。4.了解电负性的概念,知道元素的性质与电负性的关系。2.原子轨道二、原子核外电子排布规律1.能量最低原理:原子的核外电子排布遵循构造原理,使整个原子的能量处于________状态。2.泡利原理:1个原子轨道里最多只能容纳___

原子轨道ppt课件.ppt
1)电子具有波粒二象性,其运动服从量子力学的规律——不确定原理!电子的概率分布可看作一团带负电荷的“云”,称为“电子云。电子云的形状反映了电子的运动状态!5原子核外电子排布规律:1)鲍里不相容原理:每个轨道最多只能容纳两个电子,且自旋相反配对。2)能量最低原理:电子尽可能占据能量最低的轨道。1s<2s<2p<3s<3p<4s3)洪特规则:有几个简并轨道而无足够的电子填充时,必须在几个简并轨道逐一地各填充一个自旋平行的电子后,才能容纳第二个电子。思考:C、N、O、F核外电子排布?现代化学键理论是建立在量子力

电子排布及原子轨道.doc
原子轨道百科名片原子轨道(Atomicorbital)是单电子薛定谔方程的合理解ψ(x,y,z)。若用球坐标来描述这组解,即ψ(r,θ,φ)<=>R(r)·Y(θ,φ),这里R(r)是与径向分布有关的函数,称为径向分布函数,用图形描述就是原子轨道的径向分布函数;Y(θ,φ)是与角度分布有关的函数,用图形描述就是角度分布函数层次。能层(电子层)参见“电子层”原子核外运动的电子绕核运动会受到原子核的吸引,他们运动能量上的差异可用他们运动轨道离核的远近表现出来。具有动量较大的电子在离核越远的地方运动,而动量较小

原子轨道玻尔认为.ppt
编写思路从物质结构研究历程,了解化学科学的发展:1803年道尔顿原子学说1811年分子概念1860年确立原子分子论1869年发现元素周期律1903年汤姆逊模型1911卢瑟福模型1913玻尔分层排布模型19世纪中叶碳键与有机化合物分子结构研究19世纪末20世纪初微观粒子的波粒二象性量子力学模型(原子轨道)专题5物质结构的探索无止境2。从三个层次认识物质结构与性质关系(专题2-4)了解并能描述元素原子核外电子的运动状态(电子云、原子轨道)、排布规律了解原子核外电子的运动。了解s、p原子轨道的形状。认识并能说明
