
系统性大肠癌治疗PPT课件.ppt

和蔼****娘子




亲,该文档总共86页,到这已经超出免费预览范围,如果喜欢就直接下载吧~
相关资料

系统性大肠癌治疗PPT课件.ppt
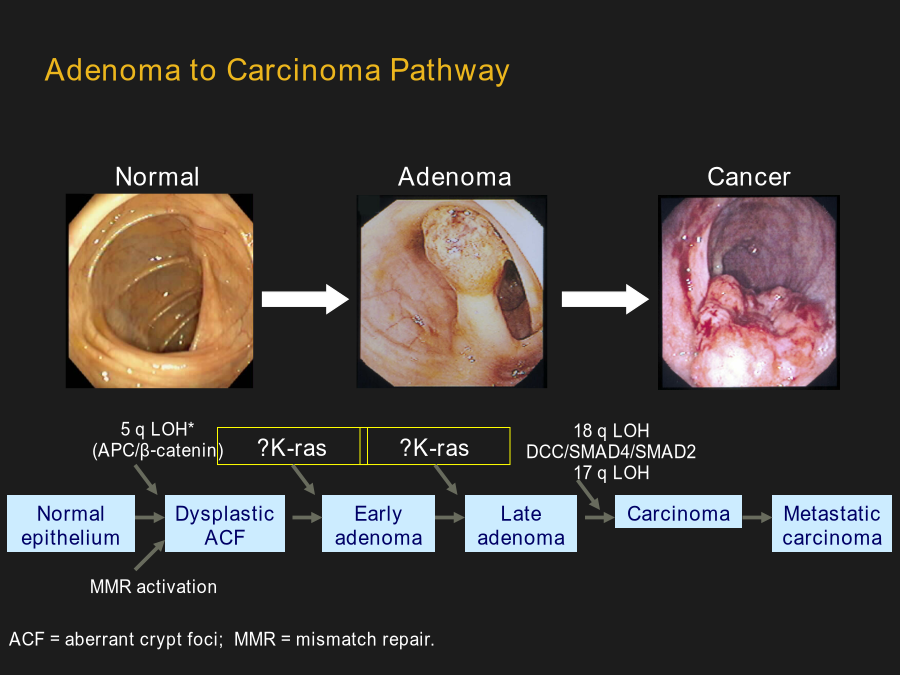
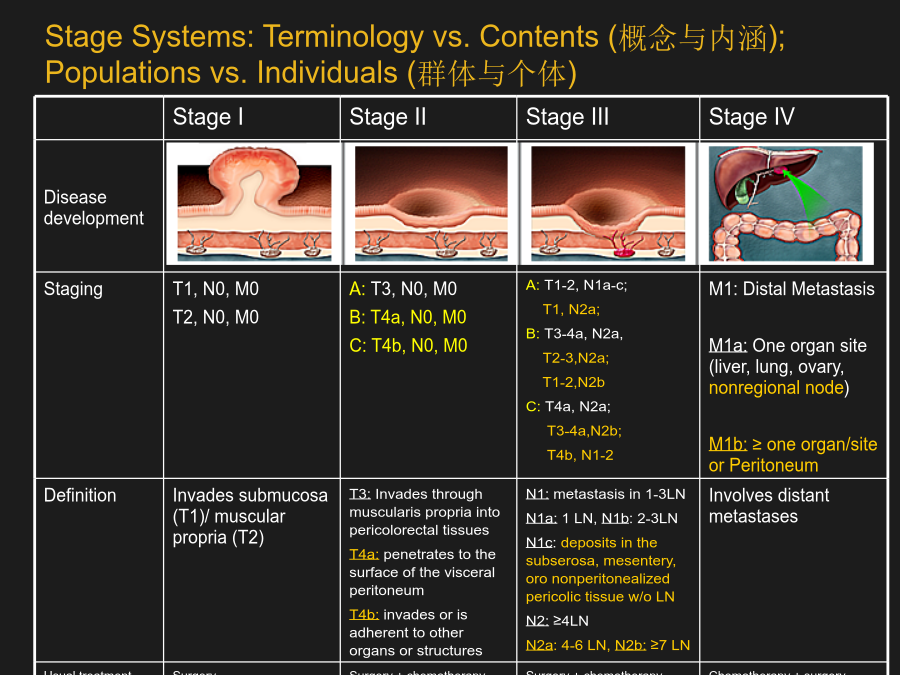
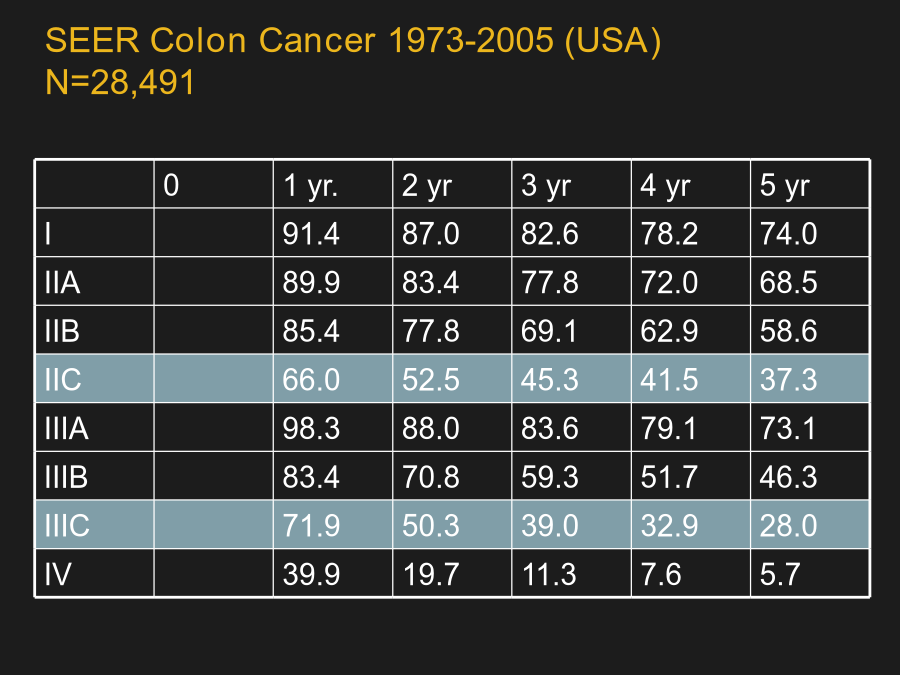
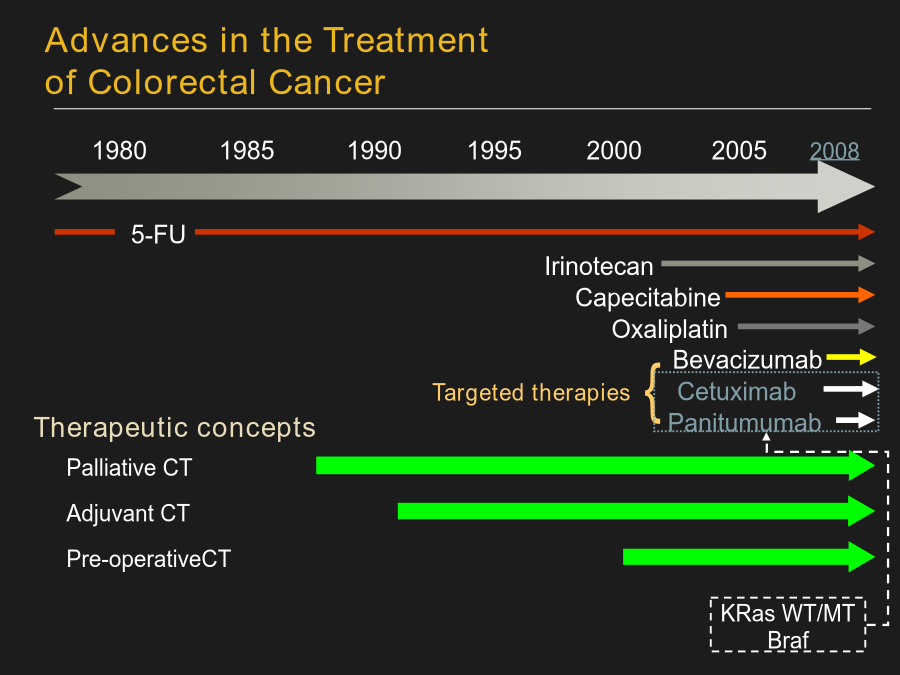
系统性大肠癌治疗AdenomatoCarcinomaPathwayStageSystems:Terminologyvs.Contents(概念与内涵);Populationsvs.Individuals(群体与个体)SEERColonCancer1973-2005(USA)N=28,491HistoryofTherapeuticRegimensfortheTreatmentofMCRCAdvancesintheTreatmentofColorectalCancerManagementofMCRC:AnEv

大肠癌的干预治疗PPT课件.ppt
医疗临床:大肠癌的干预治疗2008年美国三个学会大肠癌、腺瘤筛查的联合指南2008年美国胃肠学院指南的变化(一)2008年美国胃肠学院指南的变化(二)腺瘤——癌序列证据腺瘤临床特点自然人群腺瘤发生与发展特点腺瘤癌变规律进展期腺瘤发展为癌的比例随年龄而增加癌前病变干预治疗的效果息肉切除有效地降低大肠癌发病率肠镜检查及息肉切除是否可减少大肠癌发生美国国家息肉研究组观察各组结肠癌累计发病率大肠腺瘤性息肉内镜切除可有效的降低癌的发生切除大肠腺瘤可以降低大肠癌发病率连续普查(A组)与未普查(B组)大肠癌发生率连续1

大肠癌的诊断与治疗ppt课件.ppt
大肠癌的诊断与治疗讲课提纲一.大肠癌的发病率大肠癌的部位分布二.大肠癌的病因研究(一)大肠癌与良性肿瘤关系2、各种良性肿瘤恶变率3、大肠癌与良性肿瘤大小关系(日本资料)(二)大肠癌与慢性炎症关系(三)大肠癌与善食关系大肠癌与善食关系②(四)大肠癌与环境关系大肠癌与环境关系(五)大肠癌与其它因素三.大肠癌的临床病理(一)大肠癌的大体形态分型全国大肠癌病理研究协作组分型(1982年制定)(二)大肠癌的组织学分型全国大肠癌病理研究组分型(1982年)(三)大肠癌的临床病理分期(Dukes分期与基础)大肠癌

大肠癌的治疗进展ppt课件.ppt
大肠癌的治疗进展一.外科手术治疗二.辅助治疗

系统性真菌感染的治疗PPT课件.ppt
系统性真菌感染的治疗Q1、如何配制两性霉素B?A1:……Q2、氟康唑主要作用机理?A2:……近年来,全身性真菌感染的发病率明显地增加,引起全身性真菌感染的常见菌种中耐药菌株不断扩大。全身性真菌感染与机体的免疫功能降低有关。患者由于免疫功能严重受损,即使给予强力的经验抗真菌治疗,其预后仍是很差,病死率高达70%以上。全身性真菌感染的抗真菌治疗,已经成为临床医师们的一个棘手而紧迫的问题。定义:真菌(fungus,fungi)是一大类有细胞壁和真核结构,不分根、茎、叶,不含叶绿素,以寄生或腐生方式存在于自然界中
