
美国ICPE规定的主药和辅料的管理——David-R.-Schoneker.ppt

一条****丹淑









亲,该文档总共60页,到这已经超出免费预览范围,如果喜欢就直接下载吧~
相关资料

美国ICPE规定的主药和辅料的管理——David-R-Schoneker-.ppt
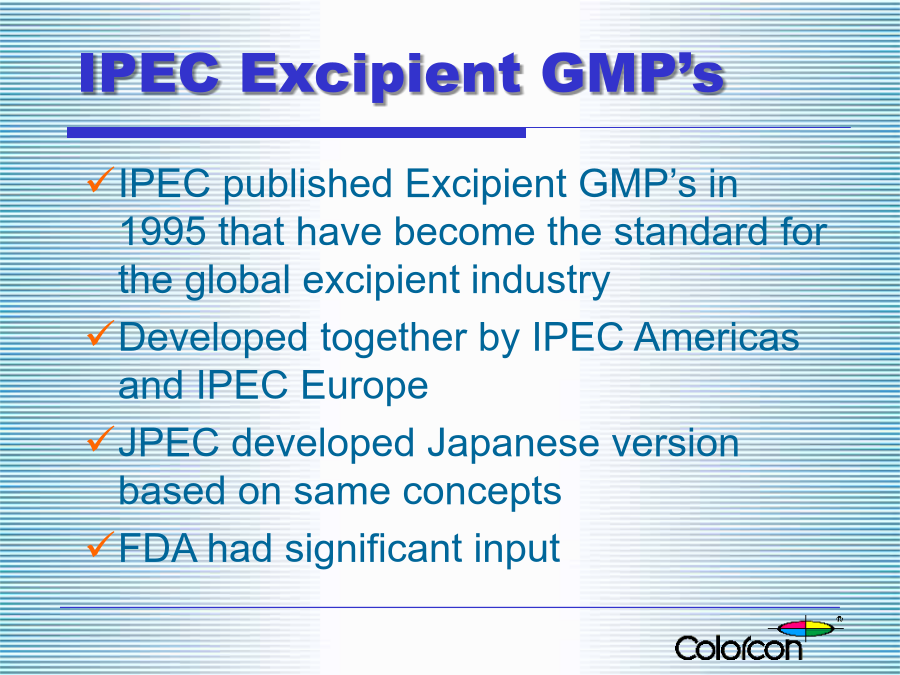
IPECActivitiesandExcipientRegulationintheUnitedStatesOutlineWhatisIPEC?WhatisIPEC?Colorcon’sInvolvementwithIPECIPECMissionBuildingBridgeswithRegulatorsExcipientGMPsintheU.S.ExcipientGMPsintheU.S.IPECExcipientGMP’sIPECExcipientGMP’sPublishedIPEC-AmericasGu

美国ICPE规定的主药和辅料的管理——David-R-Schoneker.ppt
IPECActivitiesandExcipientRegulationintheUnitedStatesOutlineWhatisIPEC?WhatisIPEC?Colorcon’sInvolvementwithIPECIPECMissionBuildingBridgeswithRegulatorsExcipientGMPsintheU.S.ExcipientGMPsintheU.S.IPECExcipientGMP’sIPECExcipientGMP’sPublishedIPEC-AmericasGu

药辅料管理.doc
关于印发《药用辅料生产质量管理规范》的通知国食药监安[2006]120号各省、自治区、直辖市食品药品监督管理局(药品监督管理局):为加强药用辅料生产的质量管理保证药用辅料质量国家局在充分征求各方面意见的基础上制定了《药用辅料生产质量管理规范》现印发给你们请结合本地实际情况参照执行。在执行中有何意见和建议请及时与我局药品安全监管司联系。国家食品药品监督管理局二○○六年三月二十八日药用辅料生产质量管理规范

原辅料仓库管理规定.docx
东莞市鸿兴食品有限公司仓库原辅料出入库作业管理规定一、目的通过对物料收/发/存等环节进行有效过程控制,以保证仓储物料的质量和数量。二、使用范围本规定适用于仓库原辅料出入库作业管理。三、货物验收作业3.1、供应商送货到厂后,仓管员应首先确认其是否携带有正确的《送货单》、《物料检验报告》和《采购订单》,三者缺一不可。资料齐全方可进入指定卸货区域等候卸货。3.2、糖浆、大豆油、白醋、三角豆等部份物料必须先通知品管抽样并初测合格后方能进行卸货作业,其它物料允许在收货入库后,再通知品管部门进行抽样检验。3.3、仓管
原辅料验收、贮存、发放管理规定.pdf
...wd...审批及颁发:部门签名日期起草仓库审核仓库批准质量受权人颁发质量保证部会审:部门签名日期部门签名日期分发:.Copy-1Copy-2Copy-3Copy-4Copy-5生产部仓库Copy-6Copy-7Copy-8Copy-9Copy-10Copy-11Copy-12Copy-11Copy-12一、目的进一步加强原辅料的管理,明确职责,特制订原辅料入库验收、贮存、发放及使用管理规定。二、范围本规定适用于原辅料的验收、贮存、发放、使用的管理。三、职责仓库负责制订本规程,部门负责人及仓库相关人员
